Abstract
For AML patients with positive pre-transplant minimal residual disease (MRD), determined by multicolor flow cytometry (MFC), haploidentical stem cell transplantation (haplo-SCT) could achieve lower CIR rates than human leukocyte antigen (HLA)-matched sibling donor transplantation (MSDT) (J Hematol Oncol. 2017;10(1):134).However, these data only provide indirect evidence supporting the idea that haplo-SCT might have a strong graft-versus-leukemia (GVL) effect compared with MSDT. Therefore, direct evidence is needed to determine whether treating AML with haplo-SCT has a stronger GVL effect.
The real time-polymerase chain reaction (RT-PCR) has greater sensitivity and specificity than MFC for detecting leukemia-specific genes, such as RUNX1/RUNX1T1, and provides a better estimation of the leukemia burden. Based on the levels of RUNX1/RUNX1T1 transcripts determined by RT-PCR, our group found that treating high risk cases with t(8;21) AML, who did not achieve major molecular remission (MMR, defined as a >3-log reduction in RUNX1/RUNX1T1 transcripts), with allogeneic stem cell transplantation (allo-SCT) could reduce the CIR compared with chemotherapy alone (22.1% vs 78.9%, P < 0.0001). These findings suggest that allo-SCT is necessary for high-risk t(8;21) AML patients, which provides an opportunity to investigate whether haplo-SCT has a superior GVL effect compared with MSDT in treating AML. Therefore, we focused on t(8;21) AML cases with positive MRD pre-transplant who underwent allogeneic SCT. The purpose of this study was to investigate and provide direct evidence that a haploidentical allograft has superior anti-leukemia effects compared with a HLA-matched sibling allograft.
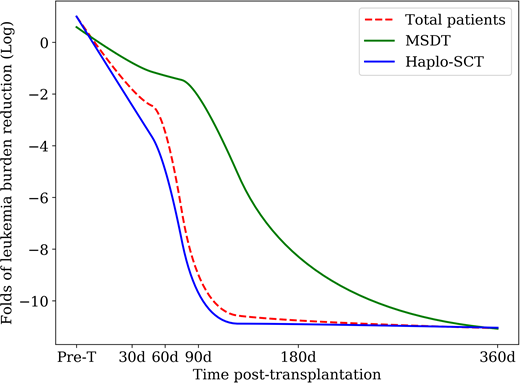
In the present study, One hundred and thirty-five t(8;21) acute myeloid leukemia (AML) patients with positive pre-transplantation minimal residual disease who underwent a haplo-SCT or MSDT were enrolled in this study. The levels of RUNX1/RUNX1T1 transcripts were monitored and quantified by real-time quantitative PCR. The 3-year cumulative incidence of relapse (CIR), non-relapse mortality (NRM), leukemia-free survival (LFS), and overall survival (OS) were 19%, 21%, 60%, and 61%, respectively. The levels of RUNX1/RUNX1T1 were significantly lower in Haplo-SCT patients compared with MSDT patients at 60 (P=0.039) and 90 (P=0.004) days after transplant (Figure 1). Compared with pre-transplant, the leukemia burden in the haplo-SCT group was significantly more reduced than in the MSDT group at 30 (P=0.068), 60 (P=0.005), 90 (P<0.0001), and 180 (P=0.032) days after transplant. The 3-year CIR rates for the haplo-SCT and MSDT patients were 14% and 28%, respectively (P=0.036). The 3-year LFS (68% vs. 47%, P=0.025) and OS (71% vs. 48%, P=0.040) after haplo-SCT were significantly higher than the rates after MSDT. In multivariate analysis, haplo-SCT was associated with a lower incidence of relapse (HR: 0.141; P=0.001) and a better LFS (HR: 0.187; P<0.0001) and OS (HR: 0.215; P=0.001). Our results provide direct evidence that a haplo-SCT has stronger graft-versus-leukemia effects than MSDT in patients with t (8;21) AML by significantly reducing the leukemia burden.
Figure 1. Kinetics of log-transformed leukemia burden reduction (evaluated by RT-PCR for RUNX1/RUNX1T1) in all patients and subgroup cases who either received haplo-SCT or MSDT, using a repeated measures ANOVA.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal